Toxins affect liver performance differently among individuals
Toxicologist, Dr Ahmed Ghallab, LiSyM Junior Group Leader at the Leibniz Research Centre for Working Environment and Human Factors (IfADo), in Dortmund, has found that chronic liver damage caused by toxins impairs the organ’s capacity to metabolize prescription drugs. This means that standard drug doses can become overdoses. Through another of his projects, Ghallab, who is also Associate Professor at the South Valley University, Egypt, was awarded the GASL (German Association of the Study of the Liver) Prize by the Foundation for Liver Research and Continuous Medical Training (YAEL) in February 2019. In this project he could explain a fundamental mechanism of acute cholestasis using intravital two-photon microscopy – a technique he developed together with his junior group.
Terms
Cholestasis – accumulation of toxic bile in the liver.
Cholestasis becomes acute when bile ducts become blocked due to gallbladder stones or compression by a tumor in the pancreas.
Intravital imaging – a technique enabling the expert to observe what is happening in cells, and between cells in living organisms.
Toxin-induced liver disease – substances can cause liver damage such as fibrosis, cirrhosis, and even cancer over a short or long period of time. It can be triggered by non-prescription and prescription drugs, herbal remedies, cleaning products, pesticides and other chemicals.
Hepatocytes – liver cells, which make up about 80% of the liver volume. They are responsible for most of the organ’s metabolic processes.
Pericentral, periportal hepatocytes – hepatocytes occurring close to the central vein are called pericentral or perivenous hepatocytes; those close to a portal vein are called periportal hepatocytes. Depending on their position between the central and portal veins, hepatocytes fulfil different functions.
Cytochrome p450 (CYP) – enzymes occurring mainly in the liver where they metabolize a multitude of substances. Many substances can also suppress or stimulate CYP activity.
PBPK models – physiologically based pharmacokinetic models describe the pathway of drugs and other substances through the body. With these models, one can extrapolate pathways of these substances in humans based on what we know from our research. This allows us to predict how an organism processes drugs, for example, and how two or more substances influence one another.
Acute cholestasis occurs when bile accumulates in the bile ducts. The toxic fluid then attacks the membranes of neighboring hepatocytes and forces its way into the cells. They perish and – in a domino effect – so too do other neighboring cells. However, after a short period, cell death ceases because the accumulated bile causes the cell walls close to the surrounding blood vessels to leak. The fluid forces its way into the bloodstream and thus drains out of the liver. “This prevents complete liver failure,” Ghallab explains. He adds that these so-called bile infarcts have been known to occur for over 100 years. “But no-one knew what caused them.” Through international cooperation, Ghallab was able to identify the mechanism. The YAEL Foundation awarded Ghallab the GASL Prize for his ‘outstanding scientific work’.

Furthermore, the toxicologist discovered that when the bile ducts become blocked, a rearrangement of these ducts takes place. “In this case many ducts appear at the damaged site, e.g. in the pericentral zone in case of toxin-induced liver cirrhosis, whereas, in a healthy liver, there are only a few bile ducts in the periportal zone.”
Ghallab’s next step is to find out whether this rearrangement benefits or harms the affected liver – by using his own technique for intravital two-photon microscopy, among others. “This method is important as it allows us to obtain video images of the liver of live mice without any prior intervention.” Other methods involve taking liver samples and preparing them, which can result in experimental artefacts. Furthermore, these methods are too slow to capture rapid biological processes. With Ghallab’s technique, up to 20 live images per second can be obtained.
Only long-term studies represent the human situation well.
The 35-year-old scientist focuses primarily on investigating the metabolic capacity of chronically damaged livers: “I am interested in how the metabolism of drugs and other substances changes in the case of toxin-induced liver damage.”
Ghallab researches these relationships using animal models and computer simulations. His team of three researchers applies toxins such as carbon tetrachloride (CCl4) to induce pathological changes in the liver. They also explore the extent to which enzyme activity and other parameters change during liver disease progression.
Ghallab explains that other research groups have conducted similar yet short-term experiments in the past, up to three months at most. There are very few studies of the later stages. “These short-term studies do not reflect the human situation,” he says. This is because disease symptoms usually only become evident in the later stages. Only then do people visit the doctor. Ghallab has extended the exposure to toxins up to one year to observe the later stages. In his studies, first a mild fibrosis in the liver develops, followed after four to six months by a severe fibroses and, later still, often cancer and cirrhosis. “The progression corresponds precisely to that in humans.”
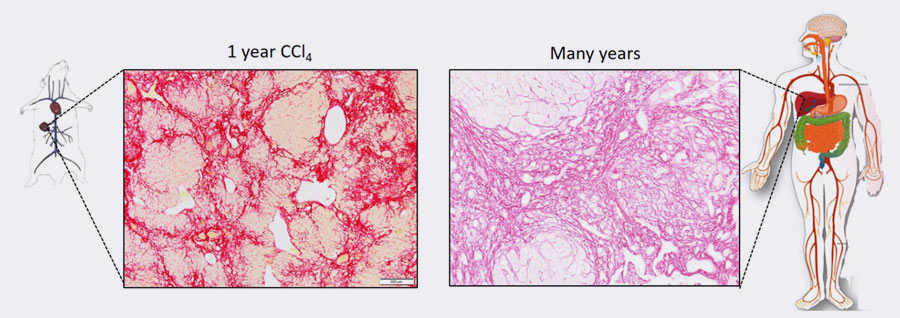
Sirius red staining showing similar collagen levels (red) in the established mouse model and in a patient with liver cirrhosis.
Chronic liver damage impairs metabolism differently in each individual. This must be considered when optimizing drug doses.
Measurements have shown that, as liver damage progresses, the activity of the crucial cytochrome p450 enzymes decreases, in some cases to the point where the activity ceases altogether. This decline in activity differs between individuals. Still, since cytochrome p450 enzymes break down many drugs, for some liver patients standard drug doses can become harmful overdoses. Next Ghallab plans to compare his data with those obtained from humans by LiSyM scientists in Pillar I. He hopes to find an indication of how drug doses can be adapted to each patient based on their actual metabolic capacity.
“Our long-term studies are unique,” says Ghallab. “We are able to make many observations that were not possible before.” For example, the spatial distribution of the cytochrome p450 enzymes in the liver, and the sensitivity of the organ changes during toxin-induced cirrhosis. In this case, the liver appears resistant to renewed doses of certain toxins. “Consequently, it becomes more prone to infections,” the toxicologist explains. This may be due to damage to the intestinal barrier after chronic liver damage, enabling intestinal bacteria to enter the liver where it causes damage.
Combining functional and spatial models
Ghallab passes his results to the computer systems biologist, Lars Küpfer, from Bayer Technology Services in Leverkusen: “We are developing a PBPK model to represent the metabolism of drugs and other substances in the human body at different stages in the progression of chronic liver damage.” Currently the model includes six test drugs, including caffeine and codeine. The aim now is to expand the model and improve its precision by including data for additional substances and repeatedly validating it. Ghallab and Küpfer plan to connect this PBPK model with the spatial models developed by LiSyM members in Pillar II.
“For me, as a scientist, the liver is an ideal organ to study,” says Ghallab, who completed his doctorate in the field of pharmacology and toxicology at the Justus-Liebig University, Giessen, before going to IfADo, where he has been leading a LiSyM Junior Group since 2015. “I definitely want to continue in research,” he stresses. He aims to refine his microscopy technique and determine the relationships between functions and malfunctions in the liver, kidney and intestines. He hopes his research will soon help people, for example, by optimizing drug doses according to the metabolic capacity of the liver of individual patients: “That would prevent some incidences of acute-on-chronic liver failure; that is acute liver failure in patients with liver disease.”
For further information on intravital imaging:
image.ifado.de.